Gallery contains 1 image
×
Photo 1 of 1
III Corps
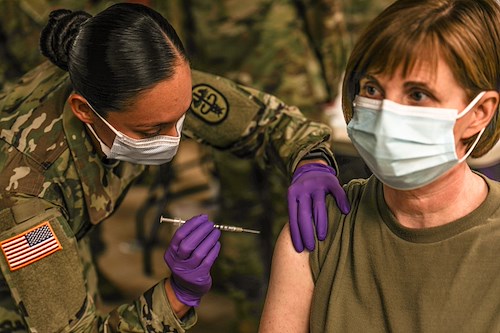
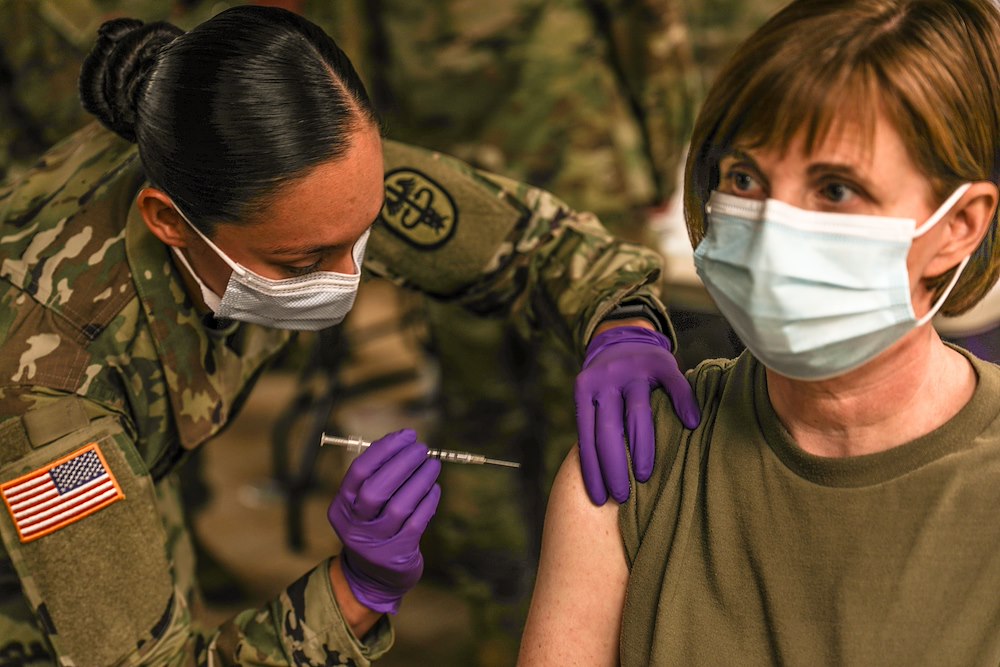
Staff Sgt. Brenda Collins, medical specialist, from Carl R. Darnall Army Medical Center administers the COVID-19 vaccination to a patient during the phase one process Dec.15, 2020 at on Fort Hood, Texas. Distribution of the vaccine will be conducted in phases based on CDC guidance. The vaccine is stored and distributed under various controls to ensure the safety of the recipient.
Photo by: Staff Sgt. Desmond Cassell
SEMBACH KASERNE, Germany – Army medical treatment facilities in Europe will soon receive an initial allocation of the Moderna COVID-19 vaccine, which recently received U.S. Food and Drug Administration approval under an Emergency Use Authorization.
The Defense Department’s initial distribution for the COVID-19 vaccine took into account the recommendations from the military services and U.S. Coast Guard in selecting the Initial distribution sites.
Frontline healthcare personnel at Landstuhl Regional Medical Center and first responders at heightened risk of exposure to individuals with COVID-19 are first in line to receive the vaccinations. Other Army MTFs in Europe will also receive the Moderna vaccine in the coming weeks, based on the same tiered distribution plan. More information on the timeline of that plan will be provided through command channels and via installation web and social media platforms as that information becomes available.
“As we work through vaccinating all of our healthcare personnel and first responders, we will also begin to look at our highly-deployable forces here in Europe, and our high-risk populations,” said Brig. Gen. Mark Thompson, Regional Health Command Europe commanding general. “After that, we’ll be able to focus on our healthy service members, civilians, families, retirees not part of the high-risk population, etc.”
According to DoD Officials, the distribution process is phase-driven to protect the department from COVID-19 as quickly as possible. These initial sites will allow DoD to validate distribution and administration processes and structures and guide senior leader decisions to increase distribution and administration as vaccine manufacturing and U.S. Centers for Disease Control and Prevention allocation permits. Initial site performance will guide follow-on site identification and the scaling of DoD distribution and administration processes.
Since the FDA approval of the vaccine was provided as an Emergency Use Authorization, the COVID vaccine will be offered on a voluntary basis.
“The FDA only authorizes the use of a COVID-19 vaccine after careful and rigorous testing and trials,” Thompson said. “We are excited to be playing a role in providing a very safe and effective vaccine to our military community. This vaccine is a vital part of our way forward to protect our people, their families and the communities where we live and work.”
As the military healthcare system in Europe begins to receive its COVID-19 vaccine allocations, host-nation healthcare facilities will also begin to offer the vaccine in the coming weeks.
“We continue to work very closely with our host-nation partners and allied nations to protect the safety of our military team and the communities where we live,” Thompson said. “Over the coming months, we expect to be able to vaccinate the majority of our military community with a DoD-supplied vaccine, but since some of our teammates receive their healthcare in the local community, I would encourage everyone to talk to their primary care provider to determine the best way to receive the vaccine.”
Thompson also emphasized that host-nation medical facilities will also be prioritizing who receives the vaccine in the early stages of the distribution process, so it could be a while before the vaccine is available to everyone.
Health officials will continue to recommend wearing masks and practicing physical distancing for everyone until the pandemic risk of COVID-19 is substantially reduced. The military community should also continue to follow military guidance and host nation rules with regards to face coverings, social/physical distancing and numbers of group gatherings.